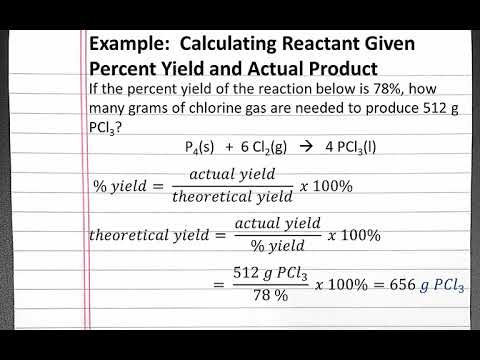
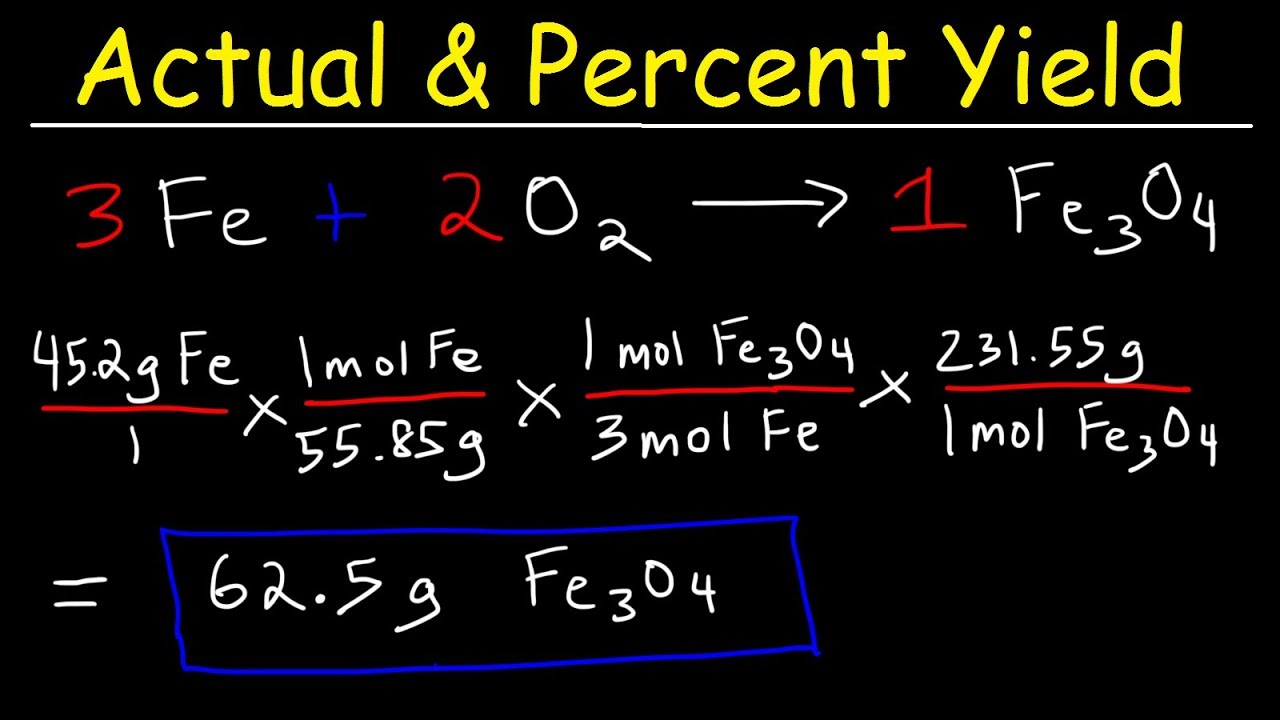
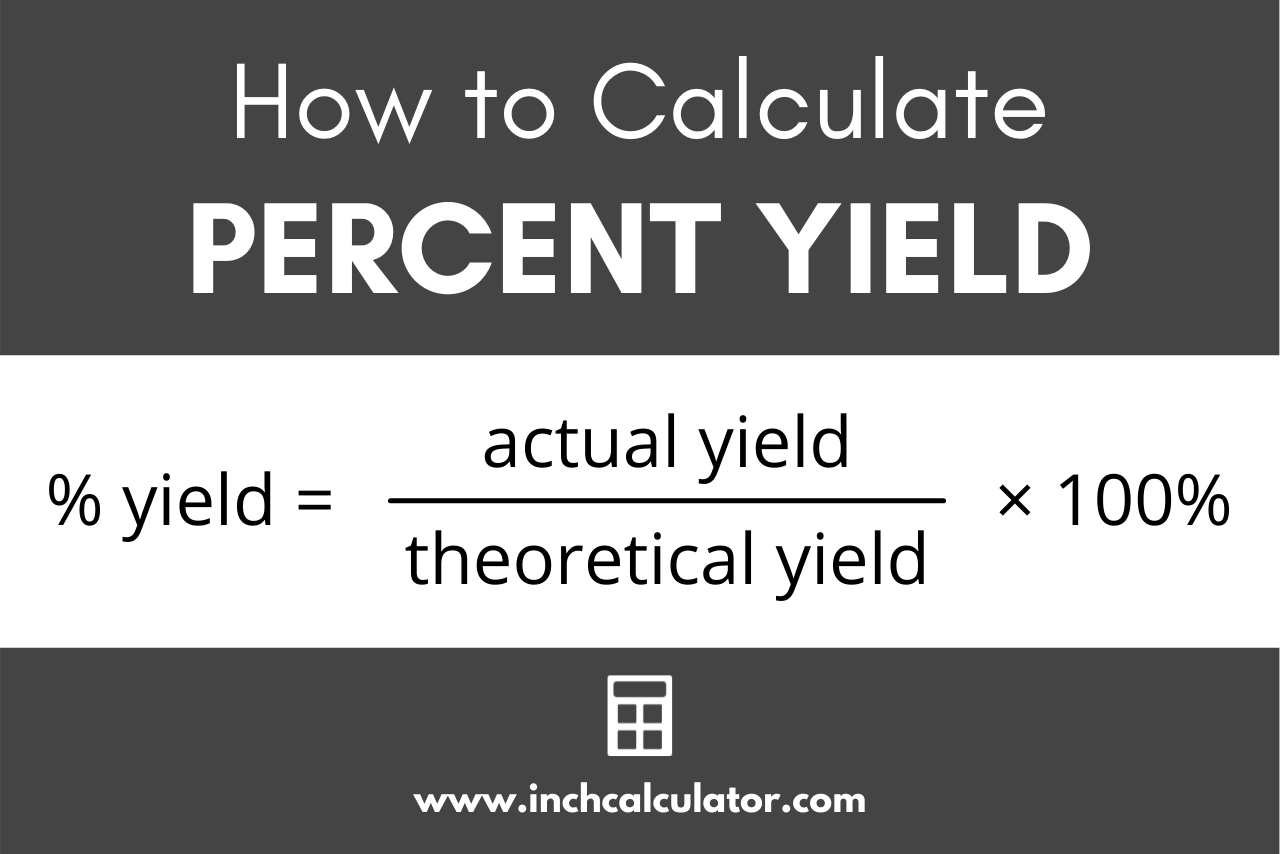
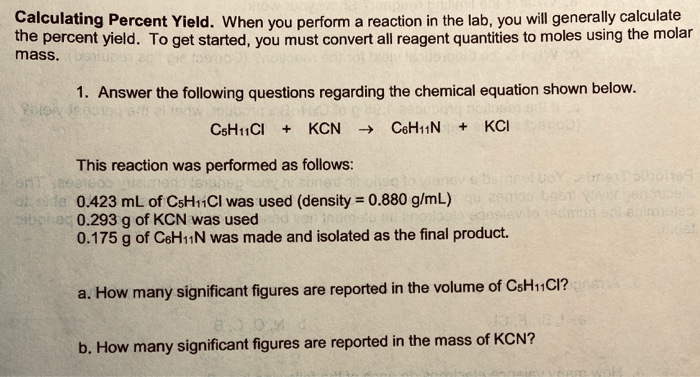
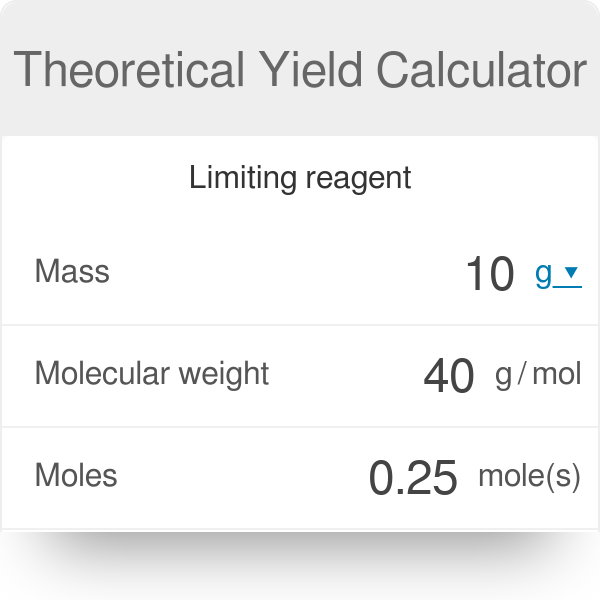
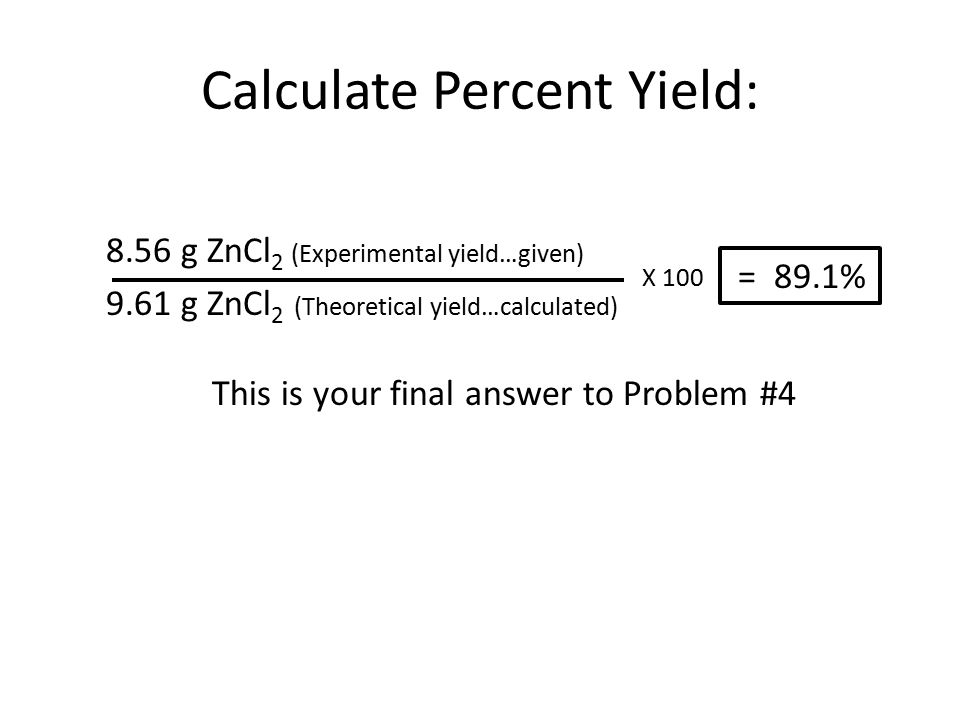
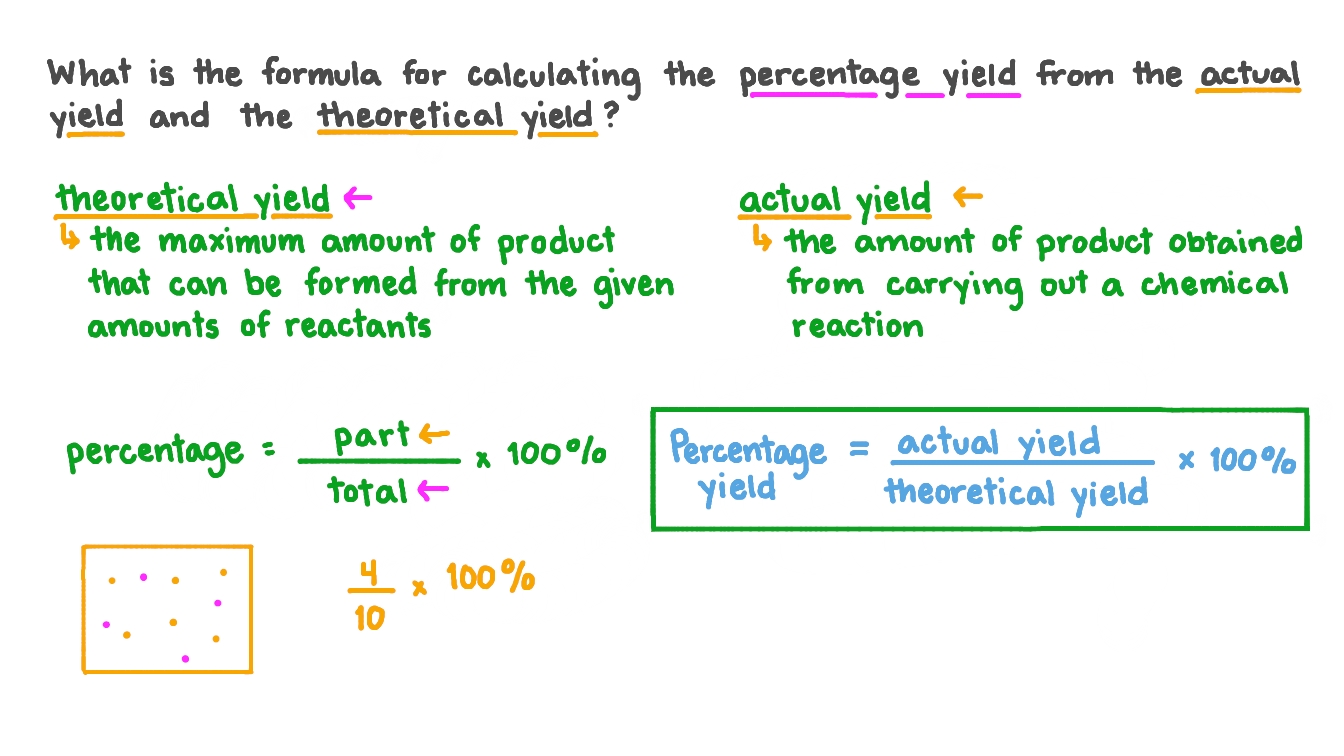
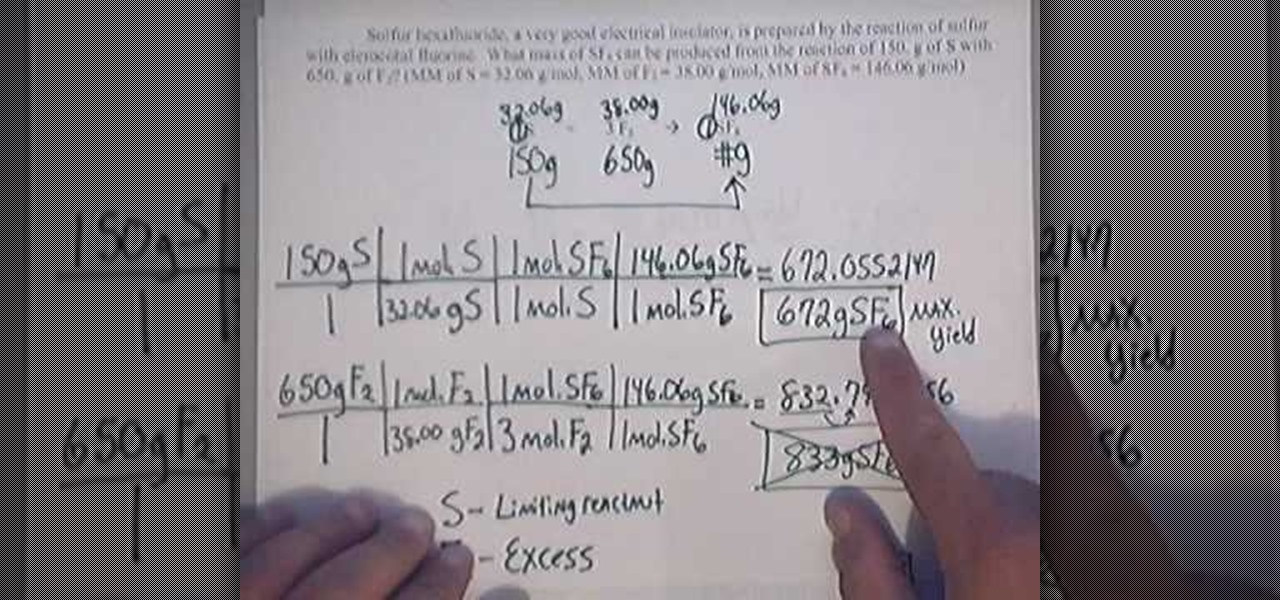Do you agree or disagree that you must always use mass when calculating the percentage yield? - Quora
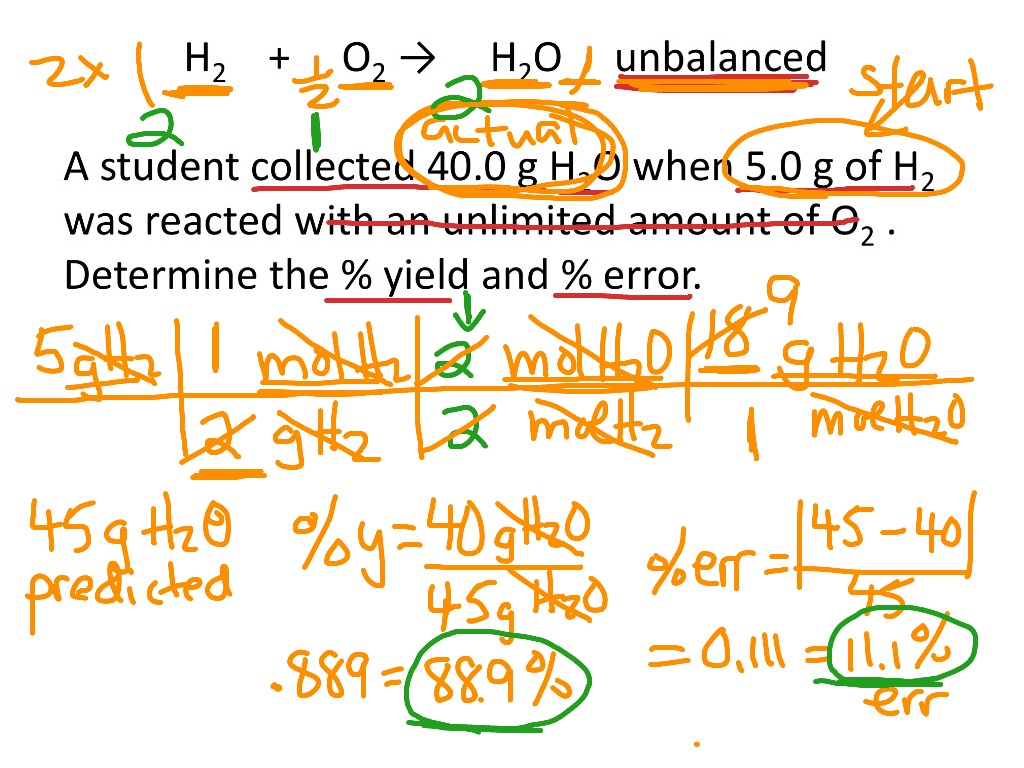
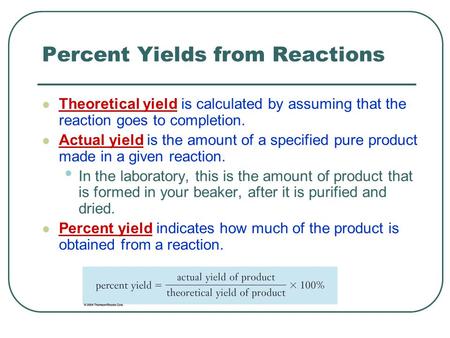
Percent Yield and Percent Error Calculations | Science, Chemistry, Percent Yield, percent error | ShowMe

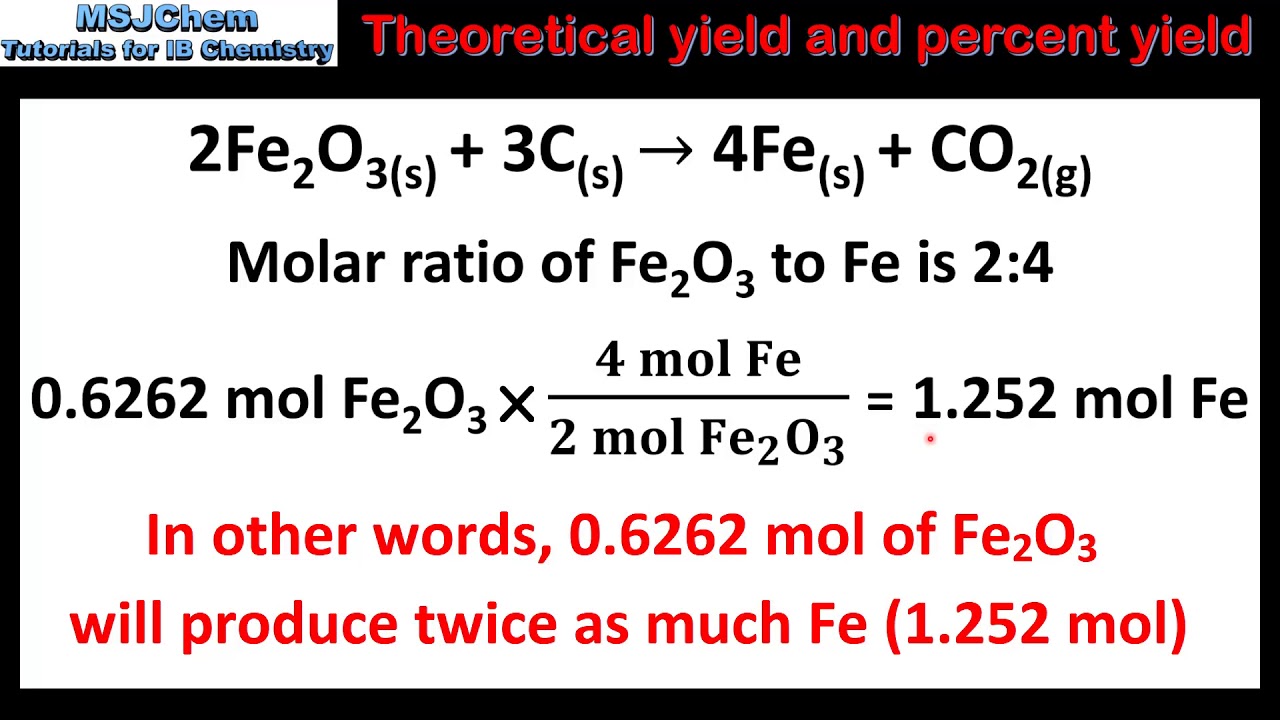
Question Video: Calculating the Percentage Yield of the Recreation of Aqueous Copper Sulfate with Zinc Metal | Nagwa
![1.5 g of Nal reacts with excess of Br2 to produce 0.412 g of NaBr, then determine the precent yield of the reaction?[atomic weight of Na = 23, Br = 80, I - 127] 2Nal + Br2→2NaBr + I2 1.5 g of Nal reacts with excess of Br2 to produce 0.412 g of NaBr, then determine the precent yield of the reaction?[atomic weight of Na = 23, Br = 80, I - 127] 2Nal + Br2→2NaBr + I2](https://dwes9vv9u0550.cloudfront.net/images/8613707/1c6b6aef-3613-4393-a328-47438145b40a.jpg)