Arrhenius equation LO- Carry out calculations involving the Arrhenius equation. So far we have looked quantitatively at how to show the effect of a concentration. - ppt download
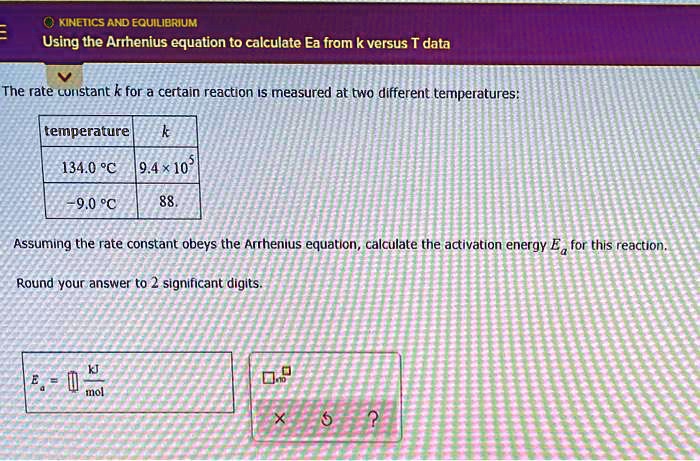
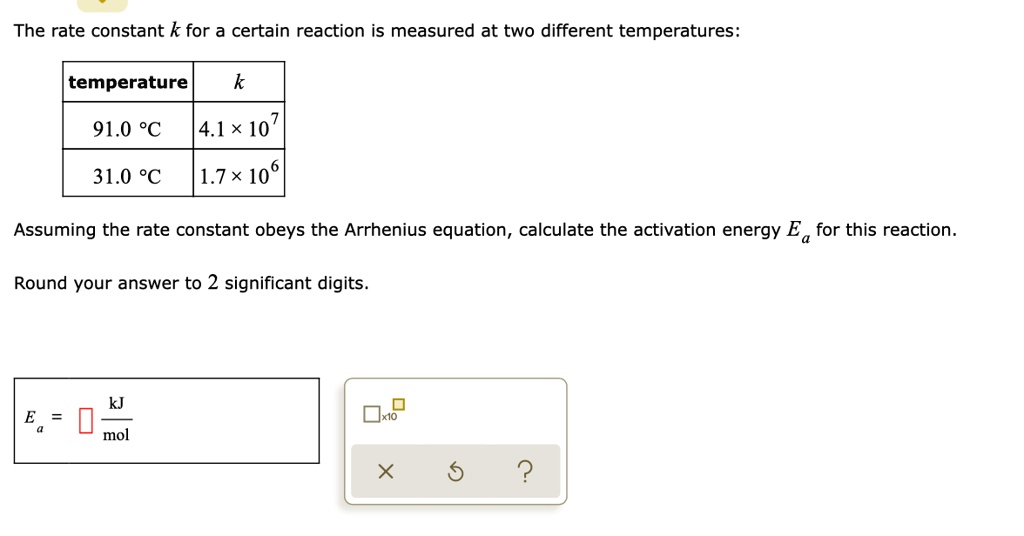
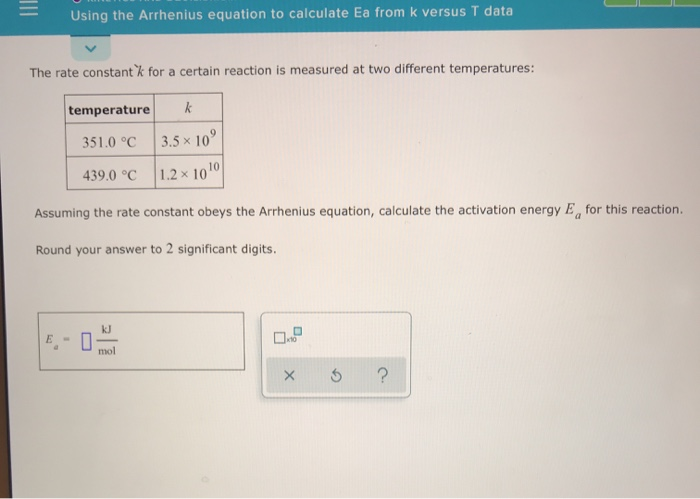
SOLVED: KINETICS AND EQUILIERIUM Using the Arrhenius equation to calculate Ea from k versus T data The rate curistant k for a certain reaction IS measured at two different temperatures: temperature 134.0 %